General Properties of d-Block Elements
General Properties of d-Block Elements: Overview
This Topic covers sub-topics such as Lanthanoid Contraction, Standard Electrode Potential of d-Block Elements, Physical Properties of d-Block Elements, Catalytic Properties of d-Block Elements and, Variable Oxidation States of d-Block Elements
Important Questions on General Properties of d-Block Elements
The correct order of magnetic moments (spin only values in B.M.) among the species involved is
Where does silver nitrate stored?
Which of the following statements are INCORRECT?
A. All the transition metals except scandium form oxides which are ionic.
B. The highest oxidation number corresponding to the group number in transition metal oxides is attained in to .
C. Basic character increases from .
D. dissolves in acids to give salts.
E. is basic but is amphoteric.
Choose the correct answer from the options given below:
The stability of is more than salts in aqueous solution due to -
For a metal ion, the calculated magnetic moment is . This metal ion has _______ number of unpaired electrons
Which of the following statements are correct?
(A) The reduction potential for iron is greater than manganese.
(B) The higher oxidation states of first row d-block elements get stabilized by oxide ion
(C) Aqueous solution of can liberate hydrogen from dilute acid
(D) Magnetic moment of is observed between
Choose the correct answer from the options given below:
Which halogen is known to cause the reaction given below?
Consider the reaction:
Find the product , that formed predominantly.
is diamagnetic in nature give reason?
The atomic number of an element is . Is this element diamagnetic or paramagnetic
Why manganese have lower boilling point than chromium?
The graph below shows the observed standard electrode potential of some transition elements.
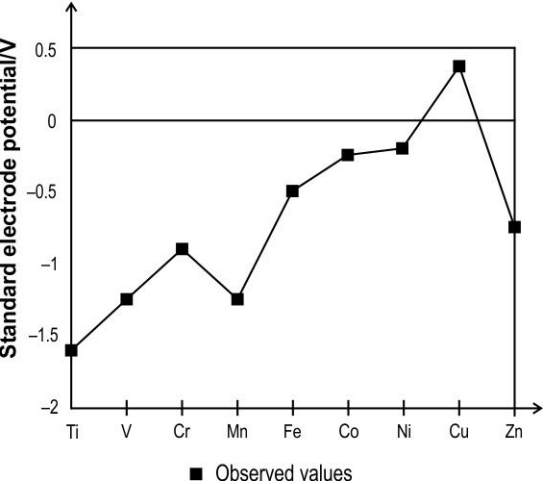
Which of the following reactions can be predicted based on the graph above?
belong to period and while belong to period. Which one is correct.
The melting point of is
Among the 3d-transition series, the
The magnetic moment of is similar to
Statement-1: salts are less stable than salts.
Statement-2: ions are formed by loss of and electrons
All halides are known, except the jodide, the reason for it is that
What will be the value of in , if the magnetic moment ?
Assertion: In general, transition metals have high melting points.
Reason: More number of electrons from '' and '' are involved in interatomic metallic bonding.
The correct option among the following is
